BREAKING Medical News! Zika Virus Can Be Used To Treat Neuroblastoma Cancer In Children!
Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 10, 2024 2 years, 3 weeks, 5 hours, 21 minutes ago
Medical News: In an unprecedented turn of events, the scientific community is abuzz with the revelation that the Zika virus, infamous for its association with birth defects, may hold the key to a groundbreaking therapy for neuroblastoma - a rare and aggressive childhood cancer. Pioneering research led by researchers from Nemours Children's Health, Florida-USA, has illuminated the potential of Zika virus injections to induce a near-total loss of neuroblastoma tumors in mouse models, bringing renewed hope to high-risk neuroblastoma patients. This
Medical News report delves into the nuances of this discovery, exploring the intricacies of neuroblastoma, the oncolytic properties of Zika virus, and the promising outcomes observed in preclinical studies.
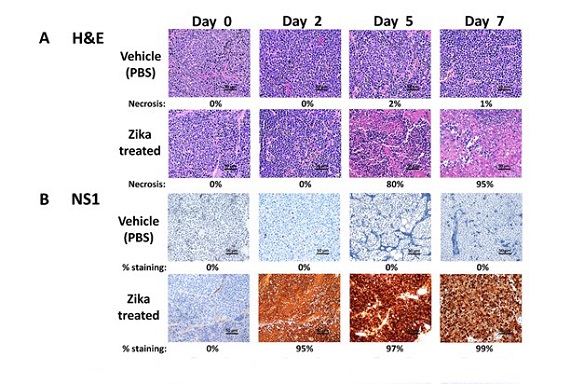 Evaluation of the Zika viral time course on neuroblastoma tumors by IHC. ZIKV-treated tumors were compared with vehicle-treated tumors at each timepoint. A, IMR-32 tumors were H&E stained at day 0, 2, 5, and 7 post-treatment. Percent necrosis is indicated under each image. B, IMR-32 tumors were stained using a Zika viral NS1 protein antibody at day 0, 2, 5, and 7 post-treatment
Understanding Neuroblastoma
Evaluation of the Zika viral time course on neuroblastoma tumors by IHC. ZIKV-treated tumors were compared with vehicle-treated tumors at each timepoint. A, IMR-32 tumors were H&E stained at day 0, 2, 5, and 7 post-treatment. Percent necrosis is indicated under each image. B, IMR-32 tumors were stained using a Zika viral NS1 protein antibody at day 0, 2, 5, and 7 post-treatment
Understanding Neuroblastoma
Neuroblastoma, constituting only 6% of childhood cancer diagnoses, emerges predominantly along the sympathetic nervous system or adrenal glands. Despite its relatively low incidence, neuroblastoma is responsible for a disproportionately high percentage - 15% - of childhood cancer-related deaths in the United States. This malignancy proves particularly challenging due to its resistance to conventional therapies, such as chemotherapy and radiation, leaving more than half of high-risk neuroblastoma patients unresponsive or prone to recurrence.
Zika Virus and its Oncolytic Potential
Recent years have witnessed a paradigm shift in cancer research, with viruses emerging as potential oncolytic agents. Among them, the Zika virus, historically known for causing birth defects in infants when pregnant women are infected, has piqued researchers' interest. The virus's ability to target the CD24 developmental protein, particularly during pregnancy, laid the groundwork for investigating its potential application in cancer therapy.
Mouse Studies and Tumor Elimination
The groundbreaking study led by Dr Tamarah Westmoreland and her team at Nemours Children's Health focused on injecting mice with neuroblastoma tumors expressing elevated levels of CD24 with Zika virus. The results were nothing short of astonishing - all mice treated with the virus exhibited a significant reduction in tumor size. In fact, the highest dosage resulted in the complete elimination of the tumor, a feat confirmed by independent pathologists at Nemours Children's Health. Equally noteworthy was the absence of tumor recurrence and the absence of symptoms related to Zika virus infection or any discernible side effects.
Human Neuroblastoma Models
To extrapolate the findings to human scenarios, the researchers developed human neuroblastoma tumor models in mice. Tumors treated wi
th Zika virus showcased a remarkable shrinkage to approximately 12% of their original mass, while those treated with a saline solution exhibited substantial growth over the same period. These compelling results underscore the potential translational impact of Zika virus therapy in human neuroblastoma cases, offering a glimmer of hope to patients facing limited treatment options.
Safety and Efficacy Considerations
While the results from the preclinical studies are highly promising, the researchers caution that the path to routine clinical use involves rigorous validation. Current efforts are focused on testing Zika virus treatment in mouse adrenal glands, closely mimicking neuroblastoma's typical location in humans. The transition to clinical trials and subsequent approval by the U.S. Food and Drug Administration (FDA) is imperative before Zika virus therapy becomes a mainstream option for neuroblastoma treatment. This meticulous approach ensures the safety and efficacy of the treatment in human subjects.
Zika Virus Background
To comprehend the significance of the recent breakthrough, it is crucial to revisit the background of the Zika virus. Initially identified in monkeys in Uganda in 1947, the virus gained global attention in 1952 when it was identified in humans. Transmitted primarily through the bite of an infected mosquito, the Zika virus was notorious for causing birth defects, including microcephaly, when pregnant women were infected. While the infection typically resulted in mild cold-like symptoms, the study in question builds upon previous research that identified the virus's potential in killing neuroblastoma cells.
Additionally, Zika virus has been explored as a potential treatment for glioblastoma, a deadly brain cancer.
The researchers investigated the oncolytic potential of Zika virus, specifically its strain MR766, in both MYCN-amplified and non-amplified neuroblastoma models.
The study reveals that Zika virus application had a rapid tumoricidal effect, leading to a nearly total loss of tumor mass without evidence of recurrence. This effect was observed in both high-risk MYCN-amplified and non-amplified models, highlighting the broad spectrum of efficacy. Intriguingly, the researchers detected the viral NS1 protein within the tumors shortly after treatment, confirming a permissive infection preceding tissue necrosis.
The role of CD24, a glycophosphatidylinositol-linked sialoglycoprotein expressed in neuroblastomas, was paramount in this oncolytic activity. Sensitivity to ZIKV killing was found to be dependent on CD24 expression, offering a potential prognostic marker for oncolytic therapy in cancers expressing this marker. The study's significance lies in its contribution to expanding the understanding of Zika virus's oncolytic targeting mechanisms and the potential for improved outcomes in a preclinical setting.
Discussion on Zika's Oncolytic Mechanism
The discussion in the study unravels the intricate oncolytic mechanisms of Zika virus, drawing comparisons with other oncolytic viruses historically repurposed for cancer treatment. While viruses like hepatitis, Epstein–Barr, West Nile, and adenovirus have been explored for their oncolytic properties, Zika virus presents unique attributes. Recent years have seen the advent of genetically engineered oncolytic viruses, such as T-VEC, a modified herpes simplex virus, gaining FDA approval for a subset of patients with melanoma.
Zika virus, as a member of the Flaviviridae family, is distinct in its clinical course, with the majority of infections being asymptomatic. The revelation of acute maternal-fetal Zika virus syndrome heightened the understanding that the virus targets neuronal progenitor cells, making it a potential oncolytic agent for cancers with a central nervous system background. The study and prior research confirmed the profound oncolytic sensitivity of neuroblastoma to Zika virus, dependent on the expression of the membrane protein CD24.
Cluster of differentiation (CD)24, recognized as a cancer stemness marker, proved to be crucial in Zika virus's oncolytic activity. The study establishes that CD24 is highly expressed in neuroblastomas and correlates with tumor differentiation and the emergence of anaplastic histologic features. Exploiting this viral sensitivity to CD24 opens avenues for utilizing it as a prognostic target in a broad range of cancers expressing CD24, many of which exhibit resistance to current clinical therapies.
Chronology of Treatment and Viral Shedding
The study provides an insightful chronology of the treatment process, offering a deeper understanding of Zika virus's oncolytic effects. Intratumoral introduction of Zika virus was chosen as the preferred method, administered within the tumor bed and margins. This approach allowed for localized viral delivery, minimizing off-target effects on unintended tissues and organs. The results were nothing short of transformative - all cases, including high-risk MYCN-amplified and non-amplified tumors, exhibited a rapid loss of tumor mass with no recurrence observed up to four weeks post-treatment.
The observation of Zika viral protein NS1 within the tumors shortly after administration marked the initiation of a tumoricidal effect. Robust viral production within the tumor correlated with a dramatic increase in necrosis, further substantiating the oncolytic activity. However, despite vigorous viral production within the tumor, shedding to the host remained minimal, emphasizing the need for further exploration into viral penetrance and replication in new hosts.
CD24 and Immunomodulation
The study hints at a potential connection between CD24 and immunomodulation within the tumor, contributing to observed differences in tumor and host outcomes. Previous research has suggested an immunosuppressive role for CD24 in tumor cells, particularly in interactions with tumor-associated macrophages. This immune evasion strategy could explain the profound differences in outcomes between tumor and host in vivo, providing additional layers to the understanding of Zika virus's oncolytic mechanisms.
Conclusion and Future Directions
In conclusion, the study unravels a new chapter in pediatric cancer therapeutics, with Zika virus emerging as a potential game-changer in the treatment of neuroblastoma. The findings not only offer hope to high-risk neuroblastoma patients but also pave the way for a paradigm shift in oncolytic virotherapy. The dependence on CD24 expression as a predictive marker expands the scope of Zika virus therapy to a myriad of CD24-expressing cancers, potentially transforming the landscape of cancer treatment.
However, the researchers emphasize the need for cautious optimism, stressing the importance of extensive validation studies to ensure the safety and efficacy of Zika virus therapy. Current efforts, including testing in mouse adrenal glands and future clinical trials, are essential milestones before regulatory approvals. The prospect of Zika virus serving as an effective bridge therapy for neuroblastoma patients holds immense promise, offering a lifeline to those facing limited treatment options.
As the scientific community eagerly awaits the unfolding of the next chapters in Zika virus therapy research, the potential implications extend beyond neuroblastoma to a broader spectrum of CD24-expressing cancers. The ongoing exploration of Zika virus's oncolytic properties signifies a significant step forward in the quest for innovative and effective cancer treatments. The collaboration between virologists, oncologists, and pediatric specialists may soon lead to transformative therapies that reshape the narrative for children and adults battling diverse forms of cancer.
The study findings were published in the peer reviewed journal: Cancer Research Communications.
https://aacrjournals.org/cancerrescommun/article/4/1/65/732575/The-Oncolytic-Activity-of-Zika-Viral-Therapy-in
For the latest
Medical News, keep on logging to Thailand Medical News.
