Study Finds That Home-Based Transcutaneous Auricular Vagus Nerve Stimulation (taVNS) Therapy Can Help In Long COVID
Source: Long COVID Jun 24, 2022 3 years, 7 months, 3 weeks, 23 hours, 59 minutes ago
A new study by researchers from the Medical University of South Carolina-USA and The City College of New York has found that home-based transcutaneous auricular vagus nerve stimulation (taVNS) therapy can help in
Long COVID.
While the COVID-19 pandemic has now impacted the world for over two years, the persistent secondary neuropsychiatric effects are still not fully understood.
Many of these “long COVID” symptoms, also referred to as post-acute sequelae of SARS-CoV-2 infection (PASC), can persist for months after infection without any effective treatments. Long COVID involves a complex heterogenous symptomology and can lead to disability and limit work. Long COVID symptoms may be due to sustained inflammatory responses and prolonged immune response after infection. Interestingly, vagus nerve stimulation (VNS) may have anti-inflammatory effects, however, until recently, VNS could not be self-administered, at-home, noninvasively.
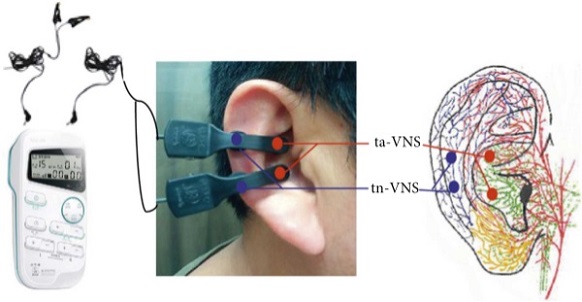
The study team created a double-blind, noninvasive transcutaneous auricular VNS (taVNS) system that can be self-administered at home with simultaneous remote monitoring of physiological biomarkers and video supervision by study staff.
The study team carried out a pilot (n = 13) randomized, sham-controlled, trial with this system for four weeks to treat nine predefined Long COVID symptoms (anxiety, depression, vertigo, anosmia, ageusia, headaches, fatigue, irritability, brain fog). No in-person patient contact was needed, with informed consent, trainings, ratings, and all procedures being conducted remotely during the pandemic (2020–2021) and equipment being shipped to individuals’ homes.
The trial was registered onClinicalTrials.gov under the identifier: NCT04638673.
https://clinicaltrials.gov/ct2/show/NCT04638673
The study findings showed that four-weeks of at-home self-administered taVNS (two, one-hour sessions daily, delivered at suprathreshold intensities) was feasible and safe.
Though the trial was not powered to determine efficacy as an intervention in a heterogenous population, the trends in the data suggest taVNS may have a mild to moderate effect in reducing mental fatigue symptoms in a subset of individuals.
The study findings demonstrate the safety and feasibility of supervised self-administered taVNS under a fully contactless protocol and suggests that future studies can safely investigate this novel form of brain stimulation at-home for a variety of neuropsychiatric and motor recovery applications.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.researchsquare.com/article/rs-1716096/v1
The study focused on assessesing the effectiveness of transcutaneous auricular vagus nerve stimulation (taVNS) in managing long coronavirus disease 2019 (COVID-19).
Although the various manifestations of COVID-19 have been extensively studied, research is still required to understand the persistent neuropsychiatric effects of the infection. These persistent symptoms are termed post-a
cute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) or long COVID and are found in patients even months after the diagnosis of COVID-19.
The study explored the feasibility and tolerability of remote-monitored taVNS and its impact on the management of symptoms related to long COVID.
The trial involved 13 individuals, including eight women, with a history of SARS-CoV-2 infection. The study procedures such as measurement of outcomes, stimulation, as well as vital monitoring were performed by the participants themselves via virtual means in the present remote-monitored at-home trial. The eligible participants were aged 18 years and above, had laboratory-confirmed SARS-CoV-2 infection, were afebrile, and reported the onset of at least one new neuropsychiatric symptom from depression, anxiety, anosmia, vertigo, ageusia, fatigue, headache, brain fog, or irritability.
The study team developed a hard-shell briefcase comprising all equipment related to the test. This included physiology monitoring and stimulation equipment as well as telecommunications devices necessary to ensure remote monitoring. After the participants received the briefcase, they were asked to charge the electronic components included in the briefcase. The participants then attended a virtual orientation session where they were instructed how to use the systems in the suitcase. After the orientation session, the team organized the first treatment visit. The participants were told they would be virtually monitored to ensure safety and compliance for the first three days of the study.
The study team developed a battery of outcomes that tracked the improvements in heterogeneous symptoms.
The key primary outcomes of the study evaluated safety and feasibility, while the secondary outcomes estimated symptom improvement of the nine above-mentioned long COVID symptoms. Feasibility was assessed during the pre-stimulation orientation visit and the initial six treatment sessions.

Safety and tolerability outcomes were measured by assessing the real-time heart rate (hr) of each participant during stimulation in the first six sessions.
The trial findings showed that during the period of initial pre-stimulation orientation, almost 91% of the participants needed assistance while using the remote hr monitoring, while all the users needed assistance with the taVNS equipment. The participants were instructed to complete two taVNS sessions every day, lasting one hour each, for four days. A compliance assessment analyzed the proportion of completed sessions out of the aggregated probable number of sessions and showed that the active and the control groups maintained a high level of compliance over a two-week randomized period.
The study data extracted from the taVNS systems revealed that the control group received sham stimulation for 52.3 minutes per session while the active cohort received active stimulation for 55.8 minutes per session.
Notably, the study team noted two incidences of mild skin irritation in the form of redness of the skin due to prolonged stimulation. On the other hand, taVNS systems did not lead to any adverse cardiac events. Heart rate observed in the first six taVNS sessions throughout the study period showed that the hr of all the participants were within the safe and normal range for adults.
.jpg)
Although the participants did not display all the nine symptoms associated with long COVID, the study team observed that the overall percentage of the symptoms could be used as a meta-indicator of the severity of long COVID. At baseline, 66% of the control group reported symptoms, while 46% of the active cohort reported symptoms.
It was found that after the blinded two-week phase, 68% of the control group showed no symptom improvement, while 31% of the active group reported a lesser number of symptoms.
After four weeks of treatment, the active and the control groups revealed 38% of the nine long COVID-19 symptoms. Notably, the control group participants who were only treated for two weeks reported an increased number of symptoms, while the active participants who were treated for four weeks showed no worsening of symptoms.
The trial findings showed the development of an entirely remote, monitored, and at-home taVNS system that was safe, highly compliant, and tolerable in the tested sample of long COVID patients. The study team believes further studies should be conducted on a larger cohort of long COVID patients.
Thailand
Medical News would like to add that there are lots of cheap and easy to use safe home-based taVNS devices originating from China that are available on various online sites ranging from Amazon to Lazada or Alibaba.
For more on
Long COVID and taVNS devices, keep on logging to Thailand
Medical News.
Read Also:
https://www.thailandmedical.news/news/spanish-researchers-claim-sars-cov-2-causes-dysfunction-of-vagus-nerve,-resulting-in-a-variety-of-conditions-associated-with-long-covid


.jpg)