Mesothelioma: New Transarterial Chemoperfusion Treatment For Mesothelioma Shows Promise For Cancer Patients
Source : Mesothelioma and Cancer Jun 15, 2020 5 years, 8 months, 1 week, 3 days, 18 hours, 15 minutes ago
Mesothelioma: According to a
cancer research abstract presented during a virtual session of the Society of Interventional Radiology's 2020 Annual Scientific Meeting held yesterday by cancer researchers from Moffitt Cancer Center in Florida and also the University of South Florida, a novel treatment for advanced mesothelioma is safe and effective and may improve the quality of life for patients who have few treatment options. The protocol called Transarterial chemoperfusion treatment for malignant pleural mesothelioma (MPM) comes with minimal side effects and shows promise for extending the lives of patients who have limited or no remaining treatment options.
https://www.sirmeeting.org/index.cfm?do=abs.viewAbs&abs=543
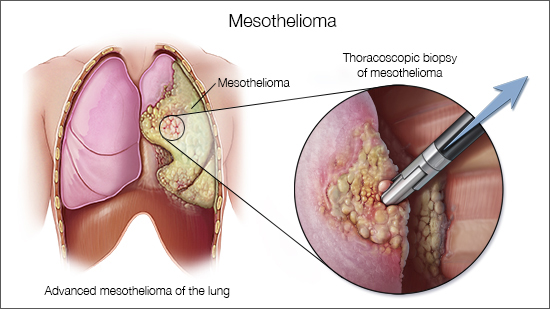
Dr Bela Kis, MD, PhD, the principal investigator on the research and an interventional radiologist at the Moffitt Cancer Center in Tampa told Thailand Medical News, "MPM is a devastating cancer of the pleura, the membranes surrounding the lungs, that is very difficult to treat. The typical survival rate of patients with stage 3 and 4 MPM is around 12 months from diagnosis; but with this new treatment, we are hoping we might be able to extend patients' lives beyond that--giving them more time with friends and family."
For the phase II clinical trial, twenty-seven patients with MPM were enrolled and underwent chemoperfusion treatment. All patients had received prior chemotherapy, many of whom received multiple lines of chemotherapy. Four of the patients had prior radiation therapy and three patients had pleurectomy. All continued to have disease progression before enrollment.
Typically, transarterial chemoperfusion delivers a relatively high concentration of drugs to diseased tissue in the lining of the lungs to maximize the treatment effect with limited side effects. Unlike other chemotherapy that is delivered intravenously and circulates through the entire body, interventional radiologists inject one-third of the chemotherapy cocktail of cisplatin, methotrexate, and gemcitabine directly into the internal mammary artery that supplies the pleura. The other two-thirds of the drugs are injected into the descending aorta, which reaches the intercostal vessels that also supply the pleura. The treatment is an outpatient procedure and typically lasts an hour, followed by a one-hour recovery.
The research interim results of the clinical trial showed a 70.3 percent disease control rate and median overall survival rate of 8.5 months from the start of the chemoperfusion treatment. The treatment was well-tolerated by patients with a major complication rate of 1.4 percent. Most side effects were relatively minor, including mild nausea and chest pain.
Dr Kis added, "We were pleasantly surprised to find that this treatment doesn't come with the same side effects of traditional intravenous chemotherapy. To see these promising results with so few side effects means we are able to make a positive impact on quality of life for these patients."
At the moment, surgery is the only truly effective treatment for MPM, but the disease must be diagnosed early. Unfortunately, only 10 to 20 percent of patients are candidates for surgery an
d often experience surgical complications.
The oncology researchers are looking to expand their study to other cancer centers with larger MPM patient populations, since the cancer is so rare. They also hope to add flexibility to the study to allow for increasing the dosage and changing the combination of medications for individual patients to determine whether either approach could further improve outcomes.
More information about the clinical trial is available at ClinicalTrials.gov, using the identifier NCT02611037.
For more about
mesothelioma or
cancer, keep on logging to Thailand Medical News
