COVID-19-Induced Kidney Damage Caused By SARS-CoV-2 Using Receptors Such As TLR-4, KIM-1/TIM-1 And CD147
Nikhil Prasad Fact checked by:Thailand Medical News Team Mar 02, 2024 1 year, 11 months, 2 weeks, 4 hours, 59 minutes ago
COVID-19 News: As the global community grapples with the ongoing COVID-19 pandemic, researchers and medical professionals are continually uncovering the myriad ways in which the SARS-CoV-2 virus impacts various organs, with the kidneys emerging as a critical focus of investigation. This
COVID-19 News report delves into the complex interplay between SARS-CoV-2 and kidney damage, exploring the involvement of receptors such as toll-like receptor 4 (TLR-4), kidney injury molecule-1/T cell immunoglobulin mucin domain 1 (KIM-1/TIM-1), and cluster of differentiation 147 (CD147). Drawing on insights from institutions like the American University of Beirut Faculty of Medicine, University Paris Saclay, INSERM-France, MatriceLab Innove Laboratory, Immeuble Les Gemeaux-France, and the University of Mississippi Medical Center-USA, we navigate through the evolving landscape of COVID-19-induced kidney abnormalities.
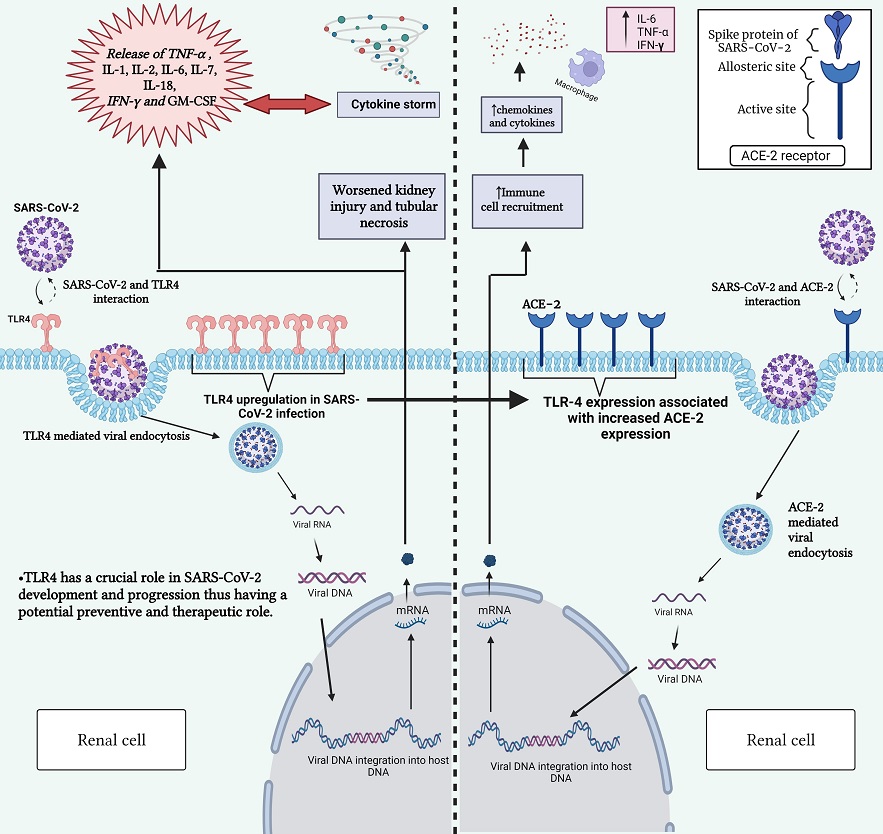 Interaction between SARS-CoV-2 and TLR-4 receptor (left) and between SARS-CoV-2 and ACE2 receptor (right). This figure highlights the role of TLR-4 in triggering immune responses upon viral recognition and the significance of ACE2 and TLR-4 receptors in mediating viral entry into host cells. Gaining insight into these interactions is vital for comprehending the mechanisms behind COVID-19 development and for devising targeted therapeutic strategies. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE2, angiotensin-converting enzyme 2; TLR-4, toll-like receptor 4; COVID-19, coronavirus disease 2019; DNA, deoxyribonucleic acid; RNA, ribonucleic acid; mRNA, messenger RNA; IL-1, interleukin-1; IL-2, interleukin-2; IL-6, interleukin-6; IL-7, interleukin-7; IL-18, interleukin-18; TNF-α, tumor necrosis factor alpha; IFN-γ, interferon gamma; GM-CSF, granulocyte-macrophage colony-stimulating factor.
The Alarming Complication: COVID-19 and Kidney Dysfunction
Interaction between SARS-CoV-2 and TLR-4 receptor (left) and between SARS-CoV-2 and ACE2 receptor (right). This figure highlights the role of TLR-4 in triggering immune responses upon viral recognition and the significance of ACE2 and TLR-4 receptors in mediating viral entry into host cells. Gaining insight into these interactions is vital for comprehending the mechanisms behind COVID-19 development and for devising targeted therapeutic strategies. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE2, angiotensin-converting enzyme 2; TLR-4, toll-like receptor 4; COVID-19, coronavirus disease 2019; DNA, deoxyribonucleic acid; RNA, ribonucleic acid; mRNA, messenger RNA; IL-1, interleukin-1; IL-2, interleukin-2; IL-6, interleukin-6; IL-7, interleukin-7; IL-18, interleukin-18; TNF-α, tumor necrosis factor alpha; IFN-γ, interferon gamma; GM-CSF, granulocyte-macrophage colony-stimulating factor.
The Alarming Complication: COVID-19 and Kidney Dysfunction
One of the most concerning complications associated with COVID-19 is the occurrence of abnormalities in kidney function. Studies indicate that acute kidney injury (AKI) is prevalent, even in cases classified as mild SARS-CoV-2 infections. The severity of kidney problems escalates dramatically in critically ill patients. A clinical perspective study involving 701 patients with moderate and severe COVID-19 symptoms revealed that 43.9% developed proteinuria, and 13% exhibited increased serum creatinine levels, blood urea nitrogen, or both, upon hospitalization. AKI, characterized by the abrupt loss of kidney function, has become a prominent extra-pulmonary complication in intensive care unit (ICU) patients, surpassing cardiac dysfunction and liver injury.
Moreover, studies underscore that the prevalence of AKI in critically ill COVID-19 patients is 20–50% higher than in those with mild and moderate symptoms. Another comprehensive study, encompassing 1248 hospitalized COVID-19 patients, reported that 43% developed AKI, establishing it as a robust predictor of 30-day mortality.
Histopathologically, SARS-CoV-2-related AKI exhibits features such a
s acute tubular necrosis and podocytopathy. The underlying mechanisms involve hemodynamic instability, disruption of the renin-angiotensin-aldosterone system, direct invasion of kidney cells by the virus, and the cytokine storm. While angiotensin-converting enzyme 2 (ACE2) was initially regarded as the primary receptor for SARS-CoV-2, recent research explores alternative pathways, shedding light on TLR-4, KIM-1/TIM-1, and CD147 as potential receptors contributing to viral entry and exacerbating kidney injury.
SARS-CoV-2-Induced Kidney Abnormalities: Insights from Autopsies and Biopsies
Investigations into autopsies of COVID-19 postmortem patients have yielded compelling evidence of SARS-CoV-2 antigen accumulation in renal epithelial tubules. Electronic microscopy studies on kidney autopsies revealed virus particles characteristic of SARS-CoV-2, primarily in podocytes and proximal tubular epithelium. Notably, another autopsy study observed the presence of SARS-CoV-2 in the glomerulus, further emphasizing the direct viral impact on the kidneys. Additionally, the detection of SARS-CoV-2 particles in urine samples adds weight to the argument that the virus can induce kidney damage directly.
Microscopic examinations of ICU patients and postmortem biopsies unveiled glomerular, acute tubular, and tubulointerstitial injuries, along with congestion in peritubular capillaries and glomeruli. Collapsing glomerulopathy, a manifestation seen in patients with non-severe respiratory symptoms, suggests the involvement of complex immune mechanisms. The immune response appears to play a pivotal role in the pathogenesis of kidney damage by COVID-19, with findings indicating the accumulation of virus antigen in the cytoplasm of tubular cells, complement membrane attack complex (C5-b9) presence, and robust deposition of CD68++ macrophages in the tubulointerstitial space.
The Prevalence of Kidney Abnormalities in COVID-19 Patients
AKI emerges as the most frequent clinical manifestation of kidney abnormalities in the context of COVID-19. Multiple studies highlight increased serum creatinine levels at admission as a negative prognostic marker for AKI. Critically ill patients often develop proteinuria and hematuria, further emphasizing the impact of SARS-CoV-2 on kidney function. Studies on 193 COVID-19 patients observed that 59% developed proteinuria, 44% exhibited hematuria, and around 10% experienced increased serum creatinine and blood urea nitrogen levels.
Alarmingly, 89% of COVID-19 patients who developed AKI did not witness an improvement in kidney function during a 3-week follow-up study.
Long-Term Consequences: AKI and COVID-19 Mortality
The repercussions of AKI in the context of COVID-19 extend beyond the acute phase. A clinical study reported that 46% of COVID-19 patients with AKI at discharge did not recover to baseline serum creatinine levels. AKI, therefore, becomes a critical factor tightly linked to increased mortality post-COVID-19. Mortality rates surge to 35% in patients with AKI compared to 6% for those without this complication.
Unraveling the Role of Toll-Like Receptor 4 (TLR-4) in Kidney Damage
Macrophages and dendritic cells, key components of the innate immune system, express Toll-like receptor 4 (TLR-4), a transmembrane protein recognized for its role in recognizing pathogen-associated molecular patterns (PAMPs) and initiating inflammatory responses. Recent studies propose that TLR-4 may serve as an alternative receptor for SARS-CoV-2, facilitating viral entry into kidney cells and triggering inflammatory cascades that culminate in renal injury.
In the context of COVID-19, TLR-4 activation leads to the release of pro-inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (IL-1β). These cytokines contribute to the cytokine storm observed in severe COVID-19 cases, exacerbating tissue damage and promoting a hyperinflammatory state that can adversely affect multiple organs, including the kidneys.
Experimental evidence supports the involvement of TLR-4 in kidney injury induced by SARS-CoV-2. Studies using TLR-4 knockout models have demonstrated attenuated renal inflammation and reduced severity of AKI following viral infection. Additionally, pharmacological inhibition of TLR-4 signaling pathways has shown promise in mitigating kidney damage in preclinical models, highlighting the therapeutic potential of targeting this receptor in COVID-19-associated kidney dysfunction.
Kidney Injury Molecule-1/T Cell Immunoglobulin Mucin Domain 1 (KIM-1/TIM-1): A Novel Pathway in COVID-19-Associated AKI
Kidney injury molecule-1 (KIM-1), also known as T cell immunoglobulin mucin domain 1 (TIM-1), is a transmembrane glycoprotein expressed at low levels in healthy kidneys but upregulated in response to various forms of renal injury. Emerging evidence suggests that KIM-1/TIM-1 may play a pivotal role in mediating kidney damage in the context of COVID-19.
SARS-CoV-2 infection induces the upregulation of KIM-1/TIM-1 expression in renal epithelial cells, potentially serving as a mechanism for viral entry and replication. Furthermore, KIM-1/TIM-1 activation triggers pro-inflammatory signaling pathways, exacerbating renal inflammation and promoting tissue injury. Experimental studies have demonstrated that blocking KIM-1/TIM-1 signaling attenuates kidney damage and improves renal function in animal models of COVID-19-associated AKI, underscoring the therapeutic potential of targeting this pathway.
Cluster of Differentiation 147 (CD147): A Key Mediator of SARS-CoV-2 Entry and Kidney Injury
Cluster of differentiation 147 (CD147), also known as extracellular matrix metalloproteinase inducer (EMMPRIN), is a transmembrane glycoprotein expressed on the surface of various cell types, including renal epithelial cells. Recent studies have implicated CD147 as a key mediator of SARS-CoV-2 entry into host cells, including kidney cells, highlighting its role in COVID-19-associated kidney injury.
SARS-CoV-2 utilizes CD147 as an alternative receptor for viral entry, facilitating viral attachment and internalization into renal epithelial cells. Subsequent viral replication within the kidney can trigger inflammatory responses, leading to tissue damage and renal dysfunction. Moreover, CD147-mediated signaling pathways promote the expression of pro-inflammatory cytokines and chemokines, further amplifying the inflammatory cascade and exacerbating kidney injury in COVID-19 patients.
Given its critical role in mediating SARS-CoV-2 entry and promoting kidney injury, CD147 represents a promising therapeutic target for the management of COVID-19-associated AKI. Strategies aimed at blocking CD147 function or inhibiting downstream signaling pathways may attenuate renal inflammation, preserve kidney function, and improve clinical outcomes in patients with COVID-19.
Several experimental approaches have shown efficacy in targeting CD147-mediated pathways in preclinical models of kidney injury. These include monoclonal antibodies directed against CD147, small molecule inhibitors of CD147-associated signaling pathways, and gene editing technologies aimed at silencing CD147 expression. Clinical trials evaluating the safety and efficacy of CD147-targeted therapies in COVID-19 patients are currently underway, offering hope for the development of novel treatments for this devastating complication.
Conclusion
The intricate relationship between SARS-CoV-2 infection and kidney damage involves a complex interplay of viral entry receptors, inflammatory mediators, and immune responses. Toll-like receptor 4 (TLR-4), kidney injury molecule-1/T cell immunoglobulin mucin domain 1 (KIM-1/TIM-1), and cluster of differentiation 147 (CD147) emerge as key players in this process, contributing to viral pathogenesis and exacerbating renal injury in COVID-19 patients.
Understanding the molecular mechanisms underlying COVID-19-associated kidney dysfunction is crucial for the development of targeted therapies aimed at preserving renal function and improving clinical outcomes in affected individuals. Future research efforts should focus on elucidating the precise roles of TLR-4, KIM-1/TIM-1, and CD147 in mediating kidney injury and exploring novel therapeutic strategies to mitigate the devastating effects of AKI in COVID-19 patients.
In conclusion, unraveling the multi-faceted nexus between COVID-19 and kidney damage mediated by TLR-4, KIM-1/TIM-1, and CD147 receptors represents a critical step towards developing effective interventions to combat this debilitating complication of SARS-CoV-2 infection.
The study findings were published in the peer reviewed journal: Front. Biosci. (IWR Press)
https://www.imrpress.com/journal/FBL/29/1/10.31083/j.fbl2901008/htm
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/thailand-medical-news-exclusive-novel-human-host-receptors-identified-for-sars-cov-2-cell-entry-and-attachment
https://www.thailandmedical.news/news/breaking-covid-19-news-sars-cov-2-found-to-also-use-human-transferrin-receptor-for-viral-entry
https://www.thailandmedical.news/news/sars-cov-2-uses-asialoglycoprotein-receptor-1-to-infect-hepatocytes
https://www.thailandmedical.news/news/covid-19-news-ukrainian-scientist-warns-that-the-alpha-7-nicotinic-acetylcholine-receptors-are-responsible-for-many-long-covid-manifestations
https://www.thailandmedical.news/news/covid-19-news-chinese-researchers-warn-that-sars-cov-2-could-be-infecting-cattle-and-other-animals-via-axl-and-nrp1-receptors
