Carrimycin Inhibits SARS-CoV-2 Replication By Decreasing The Efficiency Of Programmed-1 Ribosomal Frameshifting
Nikhil Prasad Fact checked by:Thailand Medical News Team Mar 05, 2024 1 year, 11 months, 2 weeks, 3 days, 15 hours, 40 minutes ago
COVID-19 News: The ongoing global battle against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has led to an urgent quest for effective antiviral treatments. The Chinese Academy of Medical Sciences & Peking Union Medical College has been at the forefront of this research, recently revealing a groundbreaking discovery regarding the antiviral potential of carrimycin. This macrolide antibiotic, currently in phase III clinical trials for treating COVID-19, has demonstrated a unique mechanism of action by disrupting programmed -1 ribosomal frameshifting (-1PRF), thereby impeding viral replication. This
COVID-19 News report explores the intricacies of carrimycin's antiviral prowess, shedding light on its potential as a game-changing treatment for human coronaviruses (hCoVs), including SARS-CoV-2.
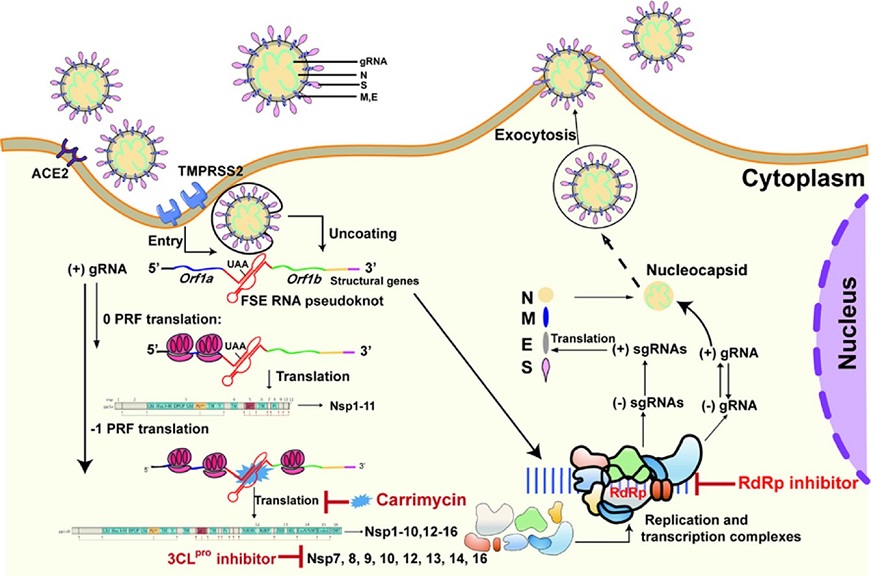 Graphical abstract
Graphical abstract
Carrimycin binds directly to the coronavirus FSE RNA pseudoknot to interrupt the translational switch of viral proteins from ORF1a to ORF1b, synergistically reducing viral replication in combination with RdRp and 3CLpro inhibitors
The Quest for Effective Antivirals
The relentless spread of SARS-CoV-2 and the emergence of new variants underscore the need for versatile antiviral drugs. While several treatments, including entry inhibitors and replicase-targeting chemicals, have received emergency use authorization, their limitations and the ever-changing nature of the virus call for innovative solutions. Carrimycin, a relatively new macrolide antibiotic approved in 2019 for bacterial infections, has emerged as a potential candidate with broad-spectrum antiviral activity. The drug is currently undergoing phase III clinical trials in multiple countries, including China and the United States.
Understanding Carrimycin's Antiviral Mechanism
Research conducted by the Chinese Academy of Medical Sciences & Peking Union Medical College delves into the intricate details of how carrimycin inhibits viral replication. Carrimycin was found effective against both α- and β-coronaviruses, including the notorious SARS-CoV-2, across various cell lines. However, the unique aspect of carrimycin's antiviral activity lies in its ability to disrupt programmed –1 ribosomal frameshifting.
The process of programmed -1 ribosomal frameshifting is crucial for the translation of viral proteins during the replication cycle of coronaviruses. Carrimycin was observed to decrease the efficiency of this frameshifting process, leading to a reduction in the translation switch from open reading frame 1a (ORF1a) to ORF1b. This interruption results in a decreased level of core components required for viral replication and transcription, effectively hindering the virus's ability to proliferate.
Experimental Validation
To validate the efficacy of carrimycin, comprehensive experiments were conducted across various cell lines and with different hCoVs, including variants of concern such as the Omicron strain. The results consistently demonstrate
d carrimycin's inhibitory activity against a broad spectrum of coronaviruses.
Notably, carrimycin disrupted viral replication in a concentration-dependent manner, affirming its potential as a versatile antiviral agent. The experiments further explored the direct interaction between carrimycin and the conserved frameshift-stimulatory element (FSE) RNA pseudoknots of coronaviruses. Surface plasmon resonance spectroscopy and isothermal titration calorimetry confirmed the specificity of this binding, highlighting carrimycin's ability to target viral RNA structures.
Unraveling the Mystery: How Carrimycin Disrupts Viral Protein Translation
One of the critical findings in the research was the elucidation of how carrimycin disrupts the translation switch from ORF1a to ORF1b. Time-of-addition experiments and entry experiments using a pseudovirus infection system provided crucial insights. Carrimycin was found to interrupt viral double-stranded RNA (dsRNA) synthesis within 8 hours post-infection (hpi), coinciding with the visualization of increased intracellular dsRNA in the untreated viral control.
However, intriguingly, in vitro assays revealed that carrimycin did not directly inhibit viral replicases, specifically the 3C-like protease (3CLpro) or the RNA-dependent RNA polymerase (RdRp). This led researchers to conclude that carrimycin likely operates before or at the stage of RNA replication, thereby disrupting the translation process.
Proteomics sequencing on infected cells further confirmed that carrimycin reduced the protein ratio of RdRp to 3CLpro. Ribosome profiling sequencing demonstrated a concentration-dependent decrease in the protein ratios of ORF1b/ORF1a-translated and RdRp/3CLpro, validating that carrimycin indeed disrupts the protein translation switch from ORF1a to ORF1b. The precision of this translation switch is controlled by programmed -1 ribosomal frameshifting, hinting at carrimycin's potential impact on this specific process.
Deciphering the Role of Programmed -1 Ribosomal Frameshifting (-1PRF)
To delve deeper into carrimycin's interference with programmed -1 ribosomal frameshifting, a dual fluorescent reporter system was established. This system relied on the -1PRF event, which is controlled by the unique viral FSE RNA signal. Carrimycin was found to reduce the efficiency of -1PRF in SARS-CoV-2, indicating its impact on this crucial step in the viral life cycle.
The inhibitory effect of carrimycin on -1PRF was comparable to that of merafloxacin, a known -1PRF inhibitor. Additionally, the research extended to test the inhibitory effect of carrimycin on other human coronaviruses, including SARS-CoV, Middle East respiratory syndrome coronavirus (MERS-CoV), hCoV-229E, hCoV-OC43, and hCoV-HKU1. The results demonstrated carrimycin's broad-spectrum activity against coronaviruses across multiple cell lines.
Direct Binding to Viral FSE RNA Pseudoknots
A crucial aspect of carrimycin's antiviral mechanism is its direct binding to viral FSE RNA pseudoknots. Surface plasmon resonance (SPR) spectroscopy experiments revealed the direct interaction between carrimycin and SARS-CoV-2 FSE RNA. Importantly, carrimycin did not bind to non-FSE-related RNA, emphasizing its specificity for viral RNA structures.
Isothermal titration calorimetry (ITC) further validated this interaction, with carrimycin demonstrating binding affinity to SARS-CoV-2 FSE RNA. The specificity extended to other coronaviral FSE RNAs, showcasing carrimycin's broad-spectrum antiviral activities. Notably, hCoV-NL63 was identified as resistant to carrimycin, supporting the specificity of this interaction.
A Rescue Experiment: Confirming the Role of FSE RNA
To confirm that the binding of carrimycin to FSE RNA is the crux of its antiviral mechanism, a rescue experiment was conducted. Huh7 cells were transfected with SARS-CoV-2 FSE RNA, infected with hCoV-229E, and treated with carrimycin. siRNA targeting the FSE RNA of hCoV-229E inhibited viral replication, underscoring the importance of FSE RNA in viral protein translation.
The inhibitory activity of carrimycin against hCoV-229E was reduced when exogenous SARS-CoV-2 FSE RNA was introduced, emphasizing the significance of FSE RNA binding. This compelling evidence further solidifies the notion that targeting FSE RNA is a promising strategy for disrupting coronaviral replication.
Synergistic Antiviral Effects and Therapeutic Potential
Carrimycin's unique mechanism of action opens doors to innovative combination therapies. Combining carrimycin with known viral replicase inhibitors, such as remdesivir, molnupiravir, and nirmatrelvir, resulted in synergistic inhibitory effects against hCoVs. These findings suggest that carrimycin's interference with viral protein translation, in conjunction with replicase inhibitors targeting other stages of the viral life cycle, could significantly enhance treatment efficacy.
Moreover, the potential synergistic effects of carrimycin with azvudine, an RdRp inhibitor approved for SARS-CoV-2 treatment in China, highlight the versatility of combination therapies in combating various coronaviruses. By targeting multiple aspects of viral replication simultaneously, these combination therapies offer a promising strategy for overcoming drug resistance and improving patient outcomes.
Implications for Future Research and Drug Development
The discovery of carrimycin's unique mechanism of action against SARS-CoV-2 and other hCoVs has profound implications for future research and drug development. Carrimycin's ability to target viral-specific RNA structures represents a significant departure from traditional antiviral approaches, which primarily focus on viral proteins.
This opens up a new frontier in antiviral drug discovery, as it expands the repertoire of targetable macromolecules beyond proteins to include RNA. By specifically targeting conserved RNA structures essential for viral replication, carrimycin exemplifies the potential of RNA-based therapeutics in combating viral infections.
Furthermore, carrimycin's broad-spectrum antiviral activity against various coronaviruses, coupled with its synergistic effects with existing antiviral drugs, underscores its potential as a cornerstone in future combination therapies. Continued research into RNA-targeting therapeutics and the development of innovative drug candidates inspired by carrimycin's mechanism of action could lead to the discovery of even more potent and broad-spectrum antiviral agents.
Conclusion
In conclusion, carrimycin emerges as a revolutionary antiviral agent with the potential to reshape the landscape of COVID-19 treatment and beyond. Its unique mechanism of action, targeting viral-specific RNA structures to disrupt programmed –1 ribosomal frameshifting, highlights a novel approach to inhibiting viral replication.
Through a series of comprehensive experiments and validations, the research team has shed light on carrimycin's remarkable antiviral properties. From its ability to inhibit viral replication across multiple cell lines to its synergistic effects with existing antiviral drugs, carrimycin offers a promising avenue for combating SARS-CoV-2 and other human coronaviruses.
As the world continues to grapple with the COVID-19 pandemic and prepares for future viral threats, carrimycin stands as a beacon of hope in the ongoing quest for effective antiviral treatments. With further research and development, carrimycin and similar RNA-targeting therapeutics could revolutionize the field of antiviral drug discovery, offering new strategies for combating viral infections and safeguarding global public health.
The study findings were published in the peer reviewed journal: Acta Pharmaceutica Sinica B.
https://www.sciencedirect.com/science/article/pii/S2211383524000765
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
