American FDA Issues Warnings About Usage Of Oximeters Especially For COVID-19 Monitoring Due To Potential Inaccuracies
Source: Pulse Oximeters And COVId-19 Feb 22, 2021 4 years, 11 months, 3 weeks, 1 day, 6 hours, 49 minutes ago
The U.S. FDA issued a new guidance last Friday warning that
pulse oximeters that have become popular during the COVID-19 pandemic for people to track their oxygen saturation may not always be accurate and that in some cases can actually lead to wrong readings and potentially put the lives of patients into high risk.
https://www.fda.gov/medical-devices/safety-communications/pulse-oximeter-accuracy-and-limitations-fda-safety-communication
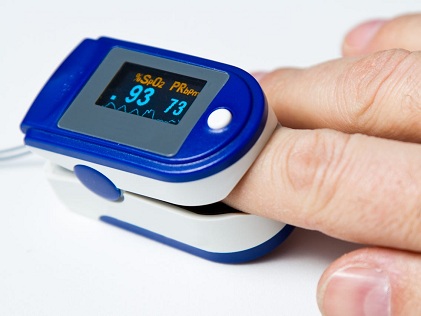
According to the new U.S. FDA safety communication guidance report, several factors can affect the accuracy of pulse oximeters, including poor circulation, skin temperature, skin thickness, current tobacco use, use of fingernail polish, and dark skin pigmentation.
Furthermore a recent report in the New England Journal of Medicine shows that Black patients may not receive accurate readings from some oximeters.
https://www.nejm.org/doi/full/10.1056/NEJMc2029240
According to that study, pulse oximeters are nearly three times more likely to miss oxygen starvation in Blacks than in whites. Among 1,609 patients treated earlier this year at the University of Michigan Hospital, in Ann Arbor, 11.7% of Blacks had an alarming arterial oxygen saturation of less than 88%, as measured directly in the blood, even though their pulse oximetry levels were in the normal range of 92% to 96%.
The oximeter devices, originally designed for people with light skin, missed low oxygen levels in only 3.6% of whites, a statistically significant difference.
When the researchers expanded their evaluation to look at data from 8,392 other patients treated at 178 intensive-care units during 2014 and 2015, they found that pulse oximeters missed low blood oxygen levels in 17.0% of Black patients versus 6.2% of whites, also a significant difference.
Lead researcher of that study, Dr Michael Sjoding of the University of Michigan Medical School told Thailand Medical News, "Given the widespread use of pulse oximetry for medical decision making, these findings have some major implications, especially during the current coronavirus disease 2019 (Covid-19) pandemic. Reliance on pulse oximetry to triage patients and adjust supplemental oxygen levels may place Black patients at increased risk for hypoxemia."
On the new U.S FDA guidance, Dr William Maisel, MD, director of the FDA's Office of Product Evaluation and Quality told Thailand Medical News, "While pulse oximeters may be useful for estimating blood oxygen levels, these devices have limitations that can result in inaccurate readings."
Dr Maisel encouraged individuals to pay attention to all of their health symptoms, especially if they experience signs of low oxygen saturation levels, such as shortness of breath or bluish coloring on their face, lips, or nails.
He added, "Patients with conditions such as COVID-19 should not rely solely on pulse oximeter measurements to monitor their health at home as they are not a substitute for a medical diagnosis by a health care provider.”
Typically a pulse oximeter, which is placed on the fingertip, uses infrared light beams to estimate the amount of oxygen in the blood and the pulse rate.
Normal oxygen saturation levels vary between 95% to 100% and can be somewhat lower in people with lung problems. Health care providers and consumers have monitored oxygen saturation during the pandemic because COVID-19 can cause levels to drop, with lower than 90% being a cause for concern.
Although consumers can buy over-the-counter oximeters in pharmacies, stores or online, but they are not intended for medical use and do not undergo U.S FDA review, according to the FDA alert.
However prescription oximeters undergo agency review and are typically used in hospitals and doctor's offices, though sometimes patients receive a prescription to use one at home.
The U.S.FDA guidance gives tips to help patients and caregivers take an accurate reading and interpret the results correctly.
Most importantly, changes or trends in measurements are more meaningful than a single measurement, according to the guidance. If patients are concerned about a pulse oximeter reading, they should contact their healthcare provider, especially if their symptoms become worse or they think they may have COVID-19.
The U.S. FDA is evaluating the current research about pulse oximeter accuracy, particularly with a focus on studies that evaluate whether the products are less accurate for people with darker skin.
Based on new findings, the U.S. FDA may update its pulse oximetry guidance and will inform the public if new information becomes available.
The U.S.FDA also encourages consumers to report any pulse oximeter issues to them directly.
Most importantly consumers around the world have to be extra careful when buying oximeters or other medical devices that are manufactured in China as most medical devices from China are of sub-standard quality with no regulatory control.
For more about
pulse oximeters, keep on logging to Thailand Medical News.
