New Cancer Drug Discovered With Help From Advanced Light Source Begins Historical Clinical Trial
Source: Thailand Medical News Dec 26, 2019 6 years, 1 month, 4 days, 1 hour, 15 minutes ago
A new investigational
cancer drug that targets tumors caused by mutations in the
KRAS gene will be evaluated in phase 2 clinical trials, following promising safety and efficacy results in preliminary human studies and excellent results in animal studies. The drug, developed by
Amgen and currently referred to as
AMG 510, is the first therapy to reach clinical trials that inhibits a mutant
KRAS protein. Errors in the
KRAS gene, which encodes a crucial cell signaling protein, are one of the most common causes of
cancer.
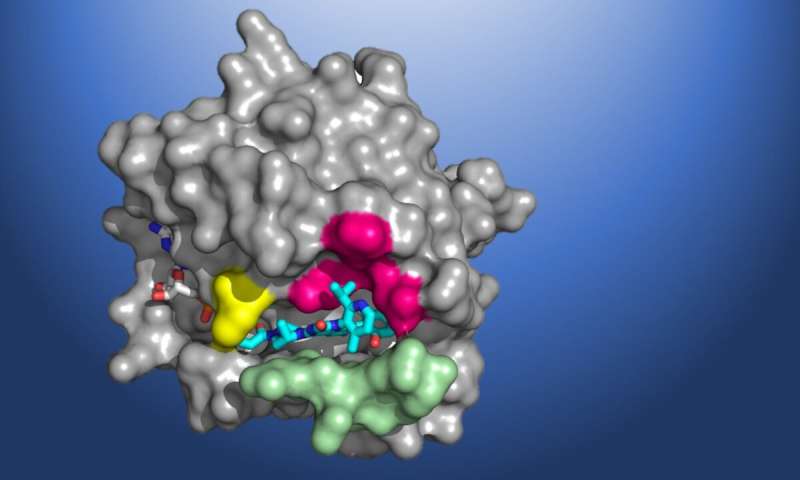 A structural map of KRAS(G12C), showing the AMG 510 molecule in the binding pocket.
A structural map of KRAS(G12C), showing the AMG 510 molecule in the binding pocket.
Amgen is a Participating Research Team member of the Berkeley Center for Structural
Biology (BCSB), which maintains and operates five of the ALS beamlines. Credit: Amgen
KRAS(G12C), the particular mutant inhibited by
AMG 510, is present in approximately 13% of lung adenocarcinomas, 3% of colorectal
cancers, and 2% of other solid tumors.
Though it plays significant role in the pathogenesis of
cancer, scientists have been unable to design
KRAS-specific therapeutics due to the shape of the protein,it has an exceptionally smooth surface with no obvious regions for a drug molecule to bind. Seeking to develop a long-sought direct inhibitor, researchers at
Amgen conducted X-ray crystallography of
KRAS(G12C) proteins at Berkeley Lab's Advanced Light Source (ALS). The high-resolution structural maps generated using the data acquired at the beamlines helped Amgen make the breakthrough discovery of a small pocket on the molecule. In subsequent studies, the beamline data allowed scientists to investigate atomic-level molecular interactions between
KRAS(G12C) and potential inhibiting compounds that bind in this pocket.
AMG 510 emerged as a very promising candidate after a multiyear drug agent optimization program.
Marc Allaire, one of the Berkeley Lab biophysicists who operate the BCSB beamlines told
Thailand Medical News, "It's rare that a compound gets all the way through the development process and becomes a drug. So, for the BCSB team, it feels great to see our small contribution finding its way to fight diseases"
Reference : Jude Canon et al, The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity, Nature (2019). DOI: 10.1038/s41586-019-1694-1
Additional Notes:
The KRAS gene identified over 30 years ago as a proto-oncogene, is one of the most frequently mutated oncogenes in human
s/cancer"> cancer. Amgen researchers first identified the novel histidine 95 (H95) groove located on an inactive
KRAS protein. Through extensive compound screening and structure-based design, AMG 510 emerged as the top investigational candidate from the optimization of a series of H95 groove-binding molecules. It is designed to irreversibly bind to
KRAS protein and permanently lock it in an inactive state, leading to inhibition of tumor cell growth in
KRAS driven tumors. In preclinical experiments,
AMG 510 demonstrated favorable potency and selectivity, and induced regression in mice bearing
KRAS mutated tumors.
There is a significant unmet need for tumor-selective therapies that minimize a negative impact on normal cells, and many patients diagnosed with
KRAS-mutated solid tumors have typically faced a challenging prognosis with limited targeted treatment options. An investigational study of
AMG 510 has seen high selectivity in non-clinical experiments, binding only to
KRAS out of more than 6,000 proteins and likely contributing to the absence of dose-limiting toxicities in the clinical study to date, supporting the potential for an encouraging safety profile."
The US FDA granted Orphan Drug Designation to
AMG 510 for previously treated metastatic non-small cell lung cancer (NSCLC) and colorectal
cancer with
KRAS G12C mutation and Fast Track Designation for previously treated metastatic NSCLC with
KRAS G12C mutation. Additional data from the ongoing Phase 1 clinical trial evaluating
AMG 510 was recently presented at the 2019 World Conference on Lung
Cancer hosted by the International Association for the Study of Lung Cancer and at the European Society for Medical Oncology 2019 Congress, both of which were held in Barcelona, Spain.
