Nikhil Prasad Fact checked by:Thailand Medica lNews Team May 26, 2024 1 year, 8 months, 2 weeks, 5 days, 13 hours, 35 minutes ago
Cardiac fibrosis, a condition characterized by the thickening and scarring of the heart tissue, is a major contributor to heart failure and other cardiac diseases. Researchers from Taipei Medical University, National Defense Medical Center, and Wan Fang Hospital in Taiwan have uncovered a crucial player in this process: a microRNA known as miR-452-5p. Their findings shed light on how this tiny molecule can regulate
Cardiac Fibrosis, offering new hope for treating heart disease.
 Graphical- Abstract - miR-452-5p: A Promising New Target in the Fight Against Cardiac Fibrosis
Understanding the Role of miR-452-5p
Graphical- Abstract - miR-452-5p: A Promising New Target in the Fight Against Cardiac Fibrosis
Understanding the Role of miR-452-5p
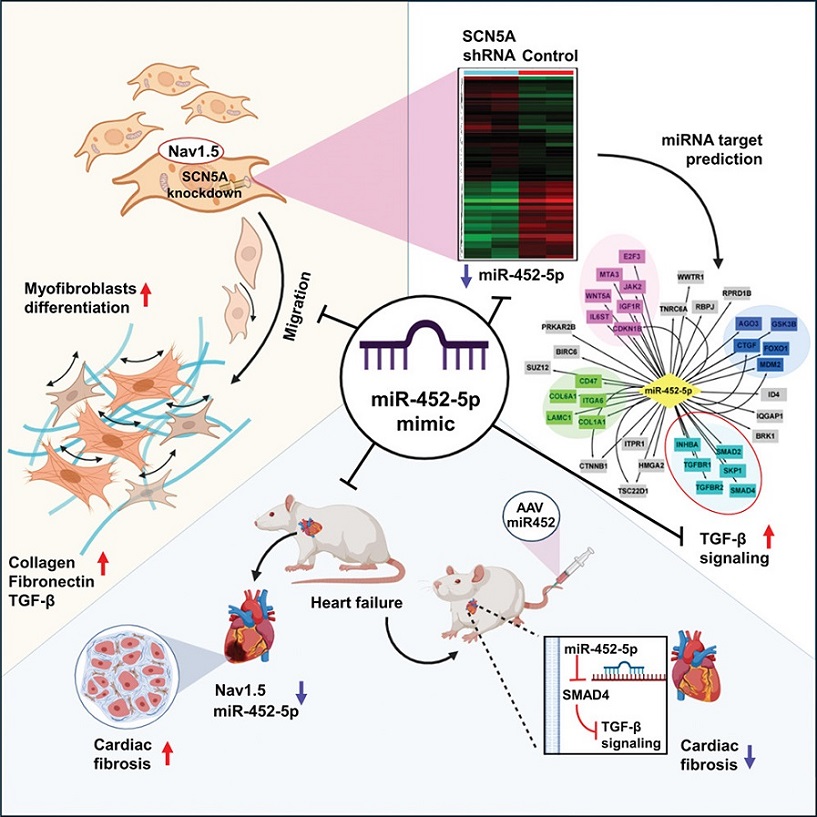
MicroRNAs (miRNAs) are small, non-coding RNA molecules that play significant roles in regulating gene expression. In this study, miR-452-5p emerged as a key regulator in the development of cardiac fibrosis, particularly in the context of a defective SCN5A gene. This gene encodes the Nav1.5 protein, a crucial component of cardiac sodium channels, which are essential for maintaining the heart's rhythm and function.
When the SCN5A gene is mutated, it results in the production of a defective Nav1.5 protein, leading to arrhythmic conditions and enhanced cardiac fibrosis. This study aimed to explore how the mutation affects cardiac fibroblasts and to identify the molecular mechanisms underlying fibrosis.
The Connection Between SCN5A and Cardiac Fibrosis
SCN5A knockdown (SCN5AKD) human cardiac fibroblasts (HCF) were used to model the effects of the SCN5A mutation. These fibroblasts exhibited increased levels of collagen, α-SMA, and fibronectin, which are markers of fibrosis. Through micro-RNA deep sequencing and qPCR analysis, researchers discovered a significant downregulation of miR-452-5p in these cells.
Bioinformatic analysis revealed that this downregulation led to the maladaptive upregulation of the transforming growth factor-β (TGF-β) signaling pathway. TGF-β is known to play a pivotal role in the development of fibrosis by promoting the differentiation of fibroblasts into myofibroblasts, which are cells that produce excessive amounts of extracellular matrix components, leading to scarring and stiffening of the heart tissue.
Targeting the TGF-β/SMAD4 Axis
The study further identified that miR-452-5p targets SMAD4, a key mediator of TGF-β signaling. Using luciferase reporter assays, the researchers confirmed that miR-452-5p directly interacts with SMAD4, inhibiting its expression and thereby attenuating the TGF-β signaling pathway.
To investigate the therapeutic potential of miR-452-5p, the researchers used a miR-452-5p mimic in SCN5AKD HCFs and an AAV9-mediated delivery system in rats with isoproterenol-induced heart failure. The results were promising: both approaches significantly reduced TGF-β signaling and fibrogenesis, leading to improved cardiac function in the heart failure model.
The Broader Implications
This study provides c
ompelling evidence that miR-452-5p plays a crucial role in regulating cardiac fibrosis through the TGF-β/SMAD4 axis. By targeting this pathway, miR-452-5p has the potential to become a novel therapeutic strategy for treating cardiac fibrosis and improving heart function in patients with SCN5A-related conditions.
The findings also highlight the importance of further exploring miRNAs in the context of cardiac diseases. These small molecules offer a new dimension to understanding the complex regulatory networks involved in heart disease and present exciting possibilities for developing targeted therapies.
Conclusion
The identification of miR-452-5p as a regulator of cardiac fibrosis marks a significant advancement in the field of cardiovascular research. By elucidating the molecular mechanisms by which miR-452-5p influences fibrosis through the TGF-β/SMAD4 axis, this study opens up new avenues for therapeutic interventions aimed at combating heart failure and other fibrotic heart conditions.
As researchers continue to unravel the complexities of miRNA-mediated regulation in cardiac diseases, the potential for developing precise and effective treatments grows ever more promising. The future of cardiac fibrosis treatment may very well hinge on the tiny but mighty miRNAs, with miR-452-5p leading the charge.
The study findings were published in the peer reviewed journal: iScience.
https://www.sciencedirect.com/science/article/pii/S2589004224013099
For the latest on
Cardiac Fibrosis, keep on logging to Thailand Medical News.
Read Also:
https://www.thailandmedical.news/news/breaking-covid-19-news-wisconsin-study-shows-that-sars-cov-2-causes-cardiac-fibrosis-many-could-die-suddenly
https://www.thailandmedical.news/news/breaking-news-german-study-find-that-30-percent-of-individuals-with-long-covid-have-non-ischemic-myocardial-fibrosis
https://www.thailandmedical.news/news/breaking-covid-19-latest-sars-cov-2-induces-immunoparalysis-of-human-host,-infects-monocytes,-macrophages-and-can-cause-fibrosis-in-post-covid-19
