COVID-19 News: E3 ligase TRIM22 Inhibits SARS-CoV-2 By Proteasomal Degradation Of NSP8
Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 28, 2024 2 years, 3 weeks, 20 hours, 44 minutes ago
COVID-19 News: The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, continues to challenge global health systems and economies. Understanding the intricate mechanisms of viral replication and host immune responses is paramount for developing effective therapeutic strategies. Among the various viral components, non-structural proteins (NSPs) play pivotal roles in viral replication, with NSP8 emerging as a critical player in the RNA-dependent RNA polymerase (RdRp) complex.
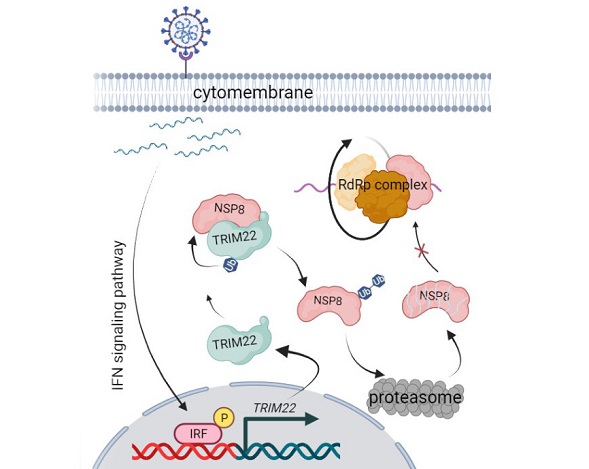 Graphical Abstract: After SARS-CoV-2 invades host cells, the released viral molecules activate the IFN signaling pathway and promote TRIM22 expression. TRIM22 binds to the NSP8 protein of SARS-CoV-2 and promotes ubiquitin proteasomal degradation of NSP8, thereby inhibiting SARS-CoV-2 replication
Graphical Abstract: After SARS-CoV-2 invades host cells, the released viral molecules activate the IFN signaling pathway and promote TRIM22 expression. TRIM22 binds to the NSP8 protein of SARS-CoV-2 and promotes ubiquitin proteasomal degradation of NSP8, thereby inhibiting SARS-CoV-2 replication
A recent study, conducted by researchers from the Southern University of Science and Technology, Shenzhen-China, Guangzhou Medical University, Guangzhou-China, and Anhui Medical University, Hefei-China, and covered in this
COVID-19 News report, provides novel insights into the instability of NSP8 and its degradation mediated by the E3 ligase tripartite motif containing 22 (TRIM22).
Discovery of NSP8 Instability and TRIM22 as its E3 Ligase
The research team embarked on a comprehensive investigation into the stability of NSP8 and its potential regulatory mechanisms. Their findings reveal a striking discovery: NSP8 exhibits a relatively unstable nature, susceptible to rapid degradation by the ubiquitin-proteasome pathway. This degradation process is mediated by TRIM22, an interferon-stimulated gene (ISG) family member, which serves as the E3 ligase responsible for catalyzing ubiquitination and subsequent degradation of NSP8. This novel revelation underscores the dynamic interplay between viral components and host immune factors during SARS-CoV-2 infection.
Exploring NSP8 Functionality and TRIM22 in Host-Virus Interactions
The study delves deeper into the multifaceted roles of NSP8 and TRIM22 in host-virus interactions. NSP8, known for its critical involvement in viral replication, emerges as a potential target for therapeutic intervention. Meanwhile, TRIM22, a versatile member of the host's innate immune system, showcases its ability to counter viral infections by targeting specific viral proteins for degradation. The elucidation of the structural domains responsible for the NSP8-TRIM22 interaction provides valuable mechanistic insights into their molecular interplay.
Ubiquitination of NSP8 and TRIM22-Mediated Inhibition of SARS-CoV-2 Replication
Upon viral invasion, the host cell's innate immune response is activated, leading to the upregulation of TRIM22 expression. TRIM22 interacts directly with NSP8 and facilitates K48-type ubiquitination at Lys97, marking NSP8 for proteasomal degradation. Through a series of meticulous experiments, the researchers elucidate the precise molecular mechanism by which TRIM22 targets NSP8, providing valuable insights into the host's antiviral defense strategies.
The
se findings underscore the critical role of host factors such as TRIM22 in modulating viral protein stability and function, highlighting the potential for host-directed therapies in combating viral infections.
IFN-α Stimulation and Species-Specific Ubiquitination
The study further explores the regulation of TRIM22 expression by interferon alpha (IFN-α) and its upregulation during SARS-CoV-2 infection. Additionally, the researchers investigated the potential species-specific ubiquitination of NSP8, revealing TRIM22's ability to target NSP8 from a broad spectrum of coronaviruses, including those of bat and pangolin origin. These findings underscore the therapeutic potential of targeting TRIM22 in combating various coronaviruses and highlight the importance of exploring host factors in the development of broad-spectrum antiviral strategies.
Implications for COVID-19 Treatment and Drug Development
The implications of these findings are profound, offering new avenues for both COVID-19 treatment and drug development. The activation of the interferon signaling pathway and subsequent upregulation of TRIM22 expression suggest a potential therapeutic role for interferons in combating SARS-CoV-2 infection. Moreover, the identification of TRIM22 as a key regulator of NSP8 stability presents an attractive target for drug development. Small molecules or compounds that modulate TRIM22 activity could potentially hinder viral replication by disrupting NSP8 stability, paving the way for the development of novel antiviral therapies.
Conclusion
In conclusion, the research conducted by the collaborative team sheds light on the intricate interplay between host and virus during SARS-CoV-2 infection. The discovery of TRIM22-mediated degradation of NSP8 unveils novel therapeutic avenues for combating COVID-19 and underscores the importance of host-directed therapies in antiviral drug development. Further research in this direction holds promise for the development of effective treatments against SARS-CoV-2 and other related coronaviruses, offering hope for a brighter future in the ongoing battle against infectious diseases.
The study findings were published in the peer reviewed journal: mBIO.
https://journals.asm.org/doi/10.1128/mbio.02320-23
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
