COVID-19 News: Doctors Report Rare Incidence Of Pilomatricoma Or Benign Skin Tumor Developing At Site Of Pfizer’s mRNA Vaccine Injection!
Nikhil Prasad Fact checked by:Thailand Medical News Team Jan 31, 2024 2 years, 2 weeks, 10 hours, 15 minutes ago
COVID-19 News: In the realm of COVID-19 vaccination, medical professionals continue to unravel the intricate web of potential side effects associated with inoculation. Amidst the myriad documented reactions, a recent case has caught the attention of the medical community - an unusual occurrence of pilomatricoma, a benign skin tumor, at the site of a Pfizer-Biontech mRNA vaccination. This perplexing case that is covered in this
COVID-19 News report, prompts a closer examination of the potential link between BNT162b2 mRNA vaccination and the development of pilomatricoma, shedding light on a rare consequence of the COVID-19 vaccination process.
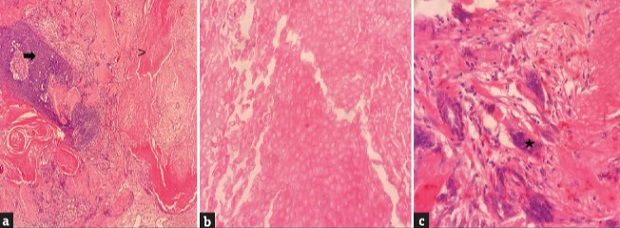 Pilomatricoma. (a) (H and EX100); solid islands of basaloid cells, which are the characteristic feature of the tumour (arrow), ghost cells (arrowhead) (b) (H and EX400) and multinucleated giant cells (asterisk) (c) (H and EX400)
The Case:
Pilomatricoma. (a) (H and EX100); solid islands of basaloid cells, which are the characteristic feature of the tumour (arrow), ghost cells (arrowhead) (b) (H and EX400) and multinucleated giant cells (asterisk) (c) (H and EX400)
The Case:
A healthy 21-year-old woman sought medical attention for an asymptomatic swelling on her left arm, which she connected to the administration of two doses of the Pfizer-Biontech BNT162b2 mRNA vaccine at the lesion site a year and a half prior. The pathology report confirmed the diagnosis of pilomatricoma, a typically slow-growing and non-cancerous skin tumor originating from hair follicle matrix cells. The presentation of pilomatricoma at the vaccination site raises questions about the potential association between the mRNA vaccine and this uncommon skin condition.
Discussion on Pilomatricoma
Pilomatricoma, also known as pilomatrixoma or Malherbe's calcified epithelioma, is a benign neoplasm commonly found on the face and neck, though it can appear elsewhere on the body. It usually presents as a single lump but can occasionally manifest as multiple growths. Despite being generally asymptomatic, its connection to trauma, including needlestick injuries and various immunizations, has been documented in medical literature. This case adds a new dimension to the existing knowledge, suggesting a potential link between pilomatricoma development and the BNT162b2 mRNA vaccine.
COVID-19 Vaccine Adverse Reactions
The ongoing global vaccination effort against COVID-19 has witnessed a spectrum of adverse reactions. Local side effects, such as pain, erythema, and swelling at the injection site, have been commonly reported. Additionally, various mucocutaneous adverse effects, including exacerbation of pre-existing dermatosis, rashes, and other skin-related issues, have been documented. This case study adds a unique perspective by presenting pilomatricoma as a potential local side effect of the COVID-19 vaccination.
Pilomatricoma and Previous Immunizations
Pilomatricomas typically appear in children and adolescents, and their exact cause remains unclear, though trauma has been implicated. Interestingly, reports exist of pilomatricoma development following different vaccines and injectable drugs. The majority of cases involve females, particularly children and young adults. The presented case aligns with this pattern, as a 21-year-old woman e
xperienced the growth of pilomatricoma following BNT162b2 mRNA vaccination.
Theories on Pilomatricoma Development
Theories propose that trauma, specifically injection site injury, might trigger pilomatricoma by impeding the apoptosis of damaged follicular epithelial cells. Persistent inflammation, wound healing, or the inoculated agent may also influence tumor development. The patient in this case reported erythema at the injection site a few weeks after the second vaccine dose, suggesting a delayed-type localized hypersensitivity reaction possibly induced by vaccine excipients. This aligns with previous reports of pilomatricoma development associated with injection site reactions, reinforcing the idea that continuous inflammation may play a role in its occurrence.
Clinical Presentation and Histopathology
Pilomatricoma typically presents as hard, bluish papules or nodules on the skin. However, it can exhibit various clinical presentations, including ulcerating, perforating, keratotic, vascular, anetodermic, and bullous types. The histopathological examination revealed a well-defined nodular lesion with ghost cells, multinucleated giant cells, and calcification, consistent with pilomatricoma. Despite its clinical appearance resembling rare subtypes, such as bullous pilomatricoma, the tumor in this case did not exhibit related histological alterations.
Need For Registry To Monitor Pilomatricoma And Other Tumor
Oncologists should set up a global registry to monitor the development and incidences of pilomatricoma and other tumor or cancers after mRNA COVID-19 vaccines as this is not the only isolated case. A number of such cases have been shared by doctors but many have not published them as cases studies due to time constraints and other factors.
Conclusion
This case study presents a compelling case of pilomatricoma possibly associated with the BNT162b2 mRNA vaccination. While the exact role of trauma and persistent inflammation in pilomatricoma development remains elusive, the presented case, along with previous reports, suggests a plausible association with the COVID-19 vaccine. The rarity of pilomatricoma underscores the importance of considering it in the differential diagnosis of nodular lesions emerging at vaccination sites. A history of injection site reactions may serve as a crucial clue for the early diagnosis of pilomatricoma, highlighting the need for further research to comprehend the intricate relationship between COVID-19 vaccines and rare skin conditions.
The cases report was published in the peer reviewed Indian Journal of Dermatology.
https://journals.lww.com/ijod/fulltext/2023/68050/pilomatricoma_growing_at_the_sars_cov_2_mrna.28.aspx
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
