Hyperactive Mutations In SARS-CoV-2 Mpro Contribute To Antiviral Drug Resistance
Nikhil Prasad Fact checked by:Thailand Medical News Team Mar 19, 2024 1 year, 11 months, 2 days, 17 hours ago
COVID-19 News: The evolution of drug resistance in rapidly evolving diseases is a significant challenge for healthcare systems worldwide. Diseases caused by viruses like SARS-CoV-2 pose particularly complex challenges due to their rapid mutation rates and ability to adapt to antiviral treatments. The emergence and spread of mutations that confer drug resistance can lead to treatment failures and have serious implications for public health. In this
COVID-19 News, we delve into the phenomenon of hyperactive mutations in the main protease (Mpro) of SARS-CoV-2 and their potential contribution to antiviral drug resistance. The study was conducted by researchers from University of Massachusetts Chan Medical School-USA and Novartis Biomedical Research-USA.
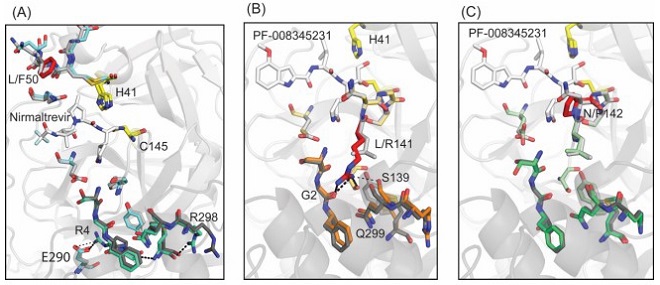 Hyperactive Mutations In SARS-CoV-2 Mpro Contribute To Antiviral Drug Resistance - Comparison of the experimentally determined X-ray crystal structures of individual hyperactive variants to WT Mpro. The mutated side-chains are colored red in all panels. (A) Overlay of WT Mpro (7RFS.PDB) with the structure of L50F. WT side chains are shown in grey or black and L50F side chains in cyan, pale green, or red. (B&C) WT Mpro overlayed with either L141R (B) or N142P (C). WT side chains are shown in grey or black and mutant side chains in red and orange (B), or red and green (C).
The Significance of Drug Resistance
Hyperactive Mutations In SARS-CoV-2 Mpro Contribute To Antiviral Drug Resistance - Comparison of the experimentally determined X-ray crystal structures of individual hyperactive variants to WT Mpro. The mutated side-chains are colored red in all panels. (A) Overlay of WT Mpro (7RFS.PDB) with the structure of L50F. WT side chains are shown in grey or black and L50F side chains in cyan, pale green, or red. (B&C) WT Mpro overlayed with either L141R (B) or N142P (C). WT side chains are shown in grey or black and mutant side chains in red and orange (B), or red and green (C).
The Significance of Drug Resistance
Drug resistance is a critical issue in the field of medicine, impacting both infectious diseases and cancers. The rapid evolution of resistance mechanisms often outpaces the development of new treatments, leading to poor health outcomes and increased mortality rates. It is estimated that drug-resistant infectious diseases could cause up to 10 million deaths globally by 2050 if effective strategies are not implemented promptly.
The COVID-19 pandemic, caused by the novel coronavirus SARS-CoV-2, has underscored the urgent need for effective antiviral therapies with reduced potential for drug resistance. The virus's genome encodes essential proteins like the main protease (Mpro), making it a prime target for antiviral drug design.
Exploring Hyperactive Mutations in SARS-CoV-2 Mpro
Mpro plays a crucial role in the viral life cycle by cleaving polyproteins into functional subunits required for viral replication and transcription. Targeting Mpro with inhibitors has been a primary strategy in developing antiviral drugs against SARS-CoV-2. Nirmatrelvir, a widely used inhibitor, binds to Mpro and prevents its enzymatic activity, thereby inhibiting viral replication.
Past studies focused on mutations that reduce inhibitor binding to Mpro. However, in this study, the study team investigated hyperactive mutations that increase Mpro's enzymatic activity. These hyperactive mutations have received less attention but can also contribute significantly to drug resistance by enhancing the virus's ability to evade inhibition.
;
Methodology and Findings
To comprehensively assess hyperactive mutations in Mpro, the study team employed a mutational scanning approach using a fluorescence resonance energy transfer (FRET)-based yeast readout. This method allowed them to analyze how individual mutations affect Mpro's enzymatic function.
Their analysis revealed hundreds of hyperactive mutations distributed throughout the Mpro structure. These mutations occurred both near and far from the active site, suggesting that factors such as protein stability and dynamics influence enzyme activity and drug resistance.
Interestingly, hyperactive mutations were observed three times more frequently than mutations reducing binding to nirmatrelvir in laboratory studies and sequenced isolates of SARS-CoV-2. This indicates that hyperactive mutations are prevalent and likely to play a crucial role in the natural evolution of drug resistance in Mpro.
Biochemical and Structural Characterization
The study team further investigated the biochemical properties of selected hyperactive mutants (T21I, L50F, and L141R) and compared them to the wild-type Mpro. These mutants exhibited increased enzymatic activity, confirming their hyperactive nature.
Structural analyses of hyperactive mutants revealed subtle changes in the protein structure, highlighting the complexity of how mutations can impact enzyme function. While some mutations led to clear structural alterations, others caused minimal changes yet significantly increased enzymatic activity.
Hotspot Analysis and Functional Implications
The study team identified several hotspot positions where multiple hyperactive mutations occurred. These hotspots suggest critical regions in Mpro's structure that are prone to hyperactivity, potentially influencing the virus's ability to evade inhibitors.
Notably, hyperactive mutations were prevalent in viruses selected for nirmatrelvir resistance in cell culture experiments. This underscores their importance in resistance evolution and their potential to confer a growth advantage to the virus in the presence of inhibitors.
Occurrence of Hyperactive Mutants in Natural Isolates
Examining the prevalence of hyperactive mutations in sequenced SARS-CoV-2 isolates revealed their presence at low levels. While these mutations are currently not widespread, their existence highlights the ongoing evolutionary dynamics of the virus and the potential for increased resistance with continued drug use.
Implications for Drug Development and Resistance Monitoring
Understanding hyperactive mutations in Mpro is crucial for designing effective antiviral drugs and monitoring resistance evolution. These mutations, although less studied than binding mutations, can significantly impact drug efficacy and contribute to treatment failures.
Future efforts should focus on developing surveillance systems to detect early signs of resistance and devising evolutionary models to predict how different drug regimens may influence resistance evolution. By staying vigilant and proactive in monitoring viral mutations, we can better combat drug resistance and improve patient outcomes in the ongoing battle against infectious diseases like COVID-19.
Conclusion
In conclusion, hyperactive mutations in SARS-CoV-2 Mpro represent a significant aspect of drug resistance evolution. Their prevalence, coupled with their potential to enhance enzymatic activity and confer resistance, underscores the need for comprehensive surveillance and proactive drug development strategies. By understanding and addressing hyperactive mutations, we can improve the effectiveness of antiviral therapies and mitigate the impact of drug-resistant viral strains on global health.
The study findings were published in the peer reviewed journal: ACS Infectious Diseases.
https://pubs.acs.org/doi/10.1021/acsinfecdis.3c00560
Thailand
Medical News would like readers to bookmark this study for future reference as it part of about 16 articles that I will be doing over time so that I can explain in detail later while referring to them as to why many repurposed drugs, supplements and also herbs and phytochemicals that we used in the first 3 years of the COVID-19 pandemic no longer works. I will not only be covering on DAAs (Direct-Acting Antivirals) but also on HDAs (Host-directed antivirals agents) as mutations in the various other accessory proteins of the virus also affects pathogenesis and way that the virus disarms key components of the host immune system and host antiviral mechanisms.
After that I will be covering another series just on SSRIs specifically on fluvoxamine and why it should never be used especially in the ongoing COVID-19 pandemic.
Though I do not expect many to really follow through..it will be interesting for those that really care and want to stay alive. If anyone has suggestions as to how I could do this series not here but on a smaller platform that is only accessible by a few..do let me know as I do not have time for stupids and worse..educated stupids!
For the latest
COVID-19 News, keep on logging to Thailand Medical News.
