BREAKING! German Study Reveals That NSP1 Protein From SARS-CoV-2 Suppresses Human Host Genes, Long Term Health Implications Expected!
Source: Medical News Sep 21, 2021 4 years, 4 months, 2 days, 19 hours, 41 minutes ago
Medical News: For a long time now, a lot of research on the SARS-CoV-2 coronavirus mode of infection and pathogenesis has only been focuses on the spike proteins with very little paid to the rest of the proteins found on the viral genome.
A new study by German scientists from Berlin Institute for Medical Systems Biology and the Max Delbrück Center for Molecular Medicine-Berlin and the Leibniz-Institut für Analytische Wissenschaften-Dortmund has found that the NSP1 protein from SARS-CoV-2 suppresses human host genes while enhancing viral RNA expression. The study findings have long term health implications for those that have been infected with the SARS-CoV-2 virus.
The study finding showed that in order to achieve its own replication and spread, the SARS-CoV-2 simultaneously depends on and subverts cellular mechanisms.
It was found that at the early stage of infection, SARS-CoV-2 expresses the viral nonstructural protein 1 (NSP1), which inhibits host translation by blocking the mRNA entry tunnel on the ribosome; this interferes with the binding of cellular mRNAs to the ribosome. Viral mRNAs, on the other hand, overcome this blockade.
The study team showed that NSP1 enhances expression of mRNAs containing the SARS-CoV-2 leader. The first stem-loop (SL1) in viral leader is both necessary and sufficient for this enhancement mechanism.
The study findings pinpoints specific residues within SL1 (three cytosine residues at the positions 15, 19 and 20) and another within NSP1 (R124) which are required for viral evasion, and thus might present promising drug targets.
The study team additionally carried out analysis of a functional interactome of NSP1 using BioID and identified components of anti-viral defense pathways.
The study findings therefore suggest a mechanism by which NSP1 inhibits the expression of host genes while enhancing that of viral RNA. This analysis helps reconcile conflicting reports in the literature regarding the mechanisms by which the virus avoids NSP1 silencing.
The study findings were published on a preprint server and are currently being peer reviewed.
https://www.biorxiv.org/content/10.1101/2021.09.13.460054v1.full
It is a known fact that to combat the effect of any novel virus or pathogen, it is important to understand the mechanism by which it infects host cells, overcomes the host immune defense mechanism, and enhances the synthesis and replication of viral proteins.
To date the most prominent preventive approach to minimize the effect of the SARS-CoV-2 coronavirus infection in human host cells has thus far been the development of vaccines. These vaccines target the viral spike protein responsible for binding to the host cells and entering the systemic circulation.
Unfortunately vaccination does not completely stop disease propagation. As SARS-CoV-2 is a novel virus, it is inevitable that it will undergo multiple rounds of mutations that produce new variants that may be resistant to existing vaccines.
Hence there is a critical need fo
r the development of drugs that target the core machinery of SARS-CoV-2 and can be used to treat infected individuals. To this end, the non-structural protein 1 (NSP1) appears to be an ideal candidate as a target.
Past research has shown NSP1 to be a conserved region in all beta-coronaviruses, the family to which SARS-CoV-2 belongs.
https://www.mdpi.com/2073-4409/10/2/300/htm
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7352669/
There has however been various conflicting past study findings about the methods by which NSP-1 invades the host system stops the synthesis of host-specific proteins and induces the synthesis of viral proteins.
This research study was meant to resolve this confusion by studying the molecular aspects of host cell entry and inhibition of host-specific cellular mechanisms.
The NSP1 protein is an important virulence factor that plays a crucial role in the pathogenicity of SARS-CoV-2 by helping the virus evade the host innate immune response. NSP1 both downregulates the expression of host genes and promotes its own propagation by neutralizing the antiviral responses of host cells to which the virus binds.
The whole genome of SARS-CoV-2 is approximately a 30 kilobase (kb) positive-stranded ribonucleic acid (RNA) virus with 5′-cap structure, 5′UTR (or leader), 3′UTR, and polyA tail.
The SARS-CoV-2 contains 10 protein-coding open reading frames (ORFs), which are parts of the genome where protein translation can occur without encountering any stop codons.
It was foun that upon cell entry, the genomic RNA (gRNA) is translated into polyprotein, which is processed into 16 non-structural proteins (NSPs). Subsequently, its gRNA serves as a template to generate a set of subgenomic mRNAs (sgRNAs) that encode for other viral proteins.
Importantly the first protein produced by coronaviruses upon infection is NSP1, which is encoded by ORF1a at the 5′ end of gRNA.
In past studies on the related SARS-CoV-1, NSP1 has been found to inhibit host immune responses by repressing expression of host transcripts and preventing full induction of interferon (IFN) and STAT1 phosphorylation, both of which are crucial for the initiation of antiviral immune responses.
https://www.pnas.org/content/103/34/12885
https://pubmed.ncbi.nlm.nih.gov/18305050/
https://pubmed.ncbi.nlm.nih.gov/23035226/
https://pubmed.ncbi.nlm.nih.gov/22855488/
https://journals.asm.org/doi/full/10.1128/JVI.00702-07
The German study team assessed the potential of NSP1 as a target by studying the detailed molecular mechanisms of its functions. To this end, they synthesized the viral genome by cloning known sequences from the SARS-CoV-1 and 2 and separating specific proteins using Western Blotting techniques.
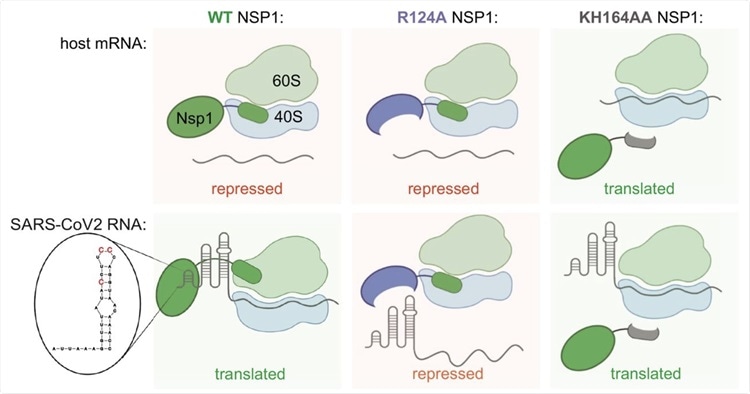 A detailed speculative model for NSP1 function in repression of host mRNA and activation of SARS-CoV-2 expression. WT NSP1 protein represses translation of host mRNA via blocking the ribosome entry tunnel. SARS-CoV-2 genomic and subgenomic RNAs escape repression by NSP1 due to SL1 in their 5′UTR, interacting with NSP1. This way viral RNA highjacks host ribosomes without competing for limiting eIFs in infected cells. In particular, positions C15, C19 and C20 are required for alleviation of NSP1 silencing. Positions K164H165 in NSP1 are required for interaction with ribosome, therefore KH164AA mutant of NSP1 does not repress either host or SARS-CoV-2 mRNA. Position R124 is required for interaction with SL1, therefore NSP1 R124A mutant represses both host and viral mRNA.
A detailed speculative model for NSP1 function in repression of host mRNA and activation of SARS-CoV-2 expression. WT NSP1 protein represses translation of host mRNA via blocking the ribosome entry tunnel. SARS-CoV-2 genomic and subgenomic RNAs escape repression by NSP1 due to SL1 in their 5′UTR, interacting with NSP1. This way viral RNA highjacks host ribosomes without competing for limiting eIFs in infected cells. In particular, positions C15, C19 and C20 are required for alleviation of NSP1 silencing. Positions K164H165 in NSP1 are required for interaction with ribosome, therefore KH164AA mutant of NSP1 does not repress either host or SARS-CoV-2 mRNA. Position R124 is required for interaction with SL1, therefore NSP1 R124A mutant represses both host and viral mRNA.
The stud team then used mutagenesis to detect the effect of different mutations in specific genes to assess their roles in immune evasion by SARS-CoV-2 NSP1. Reporter assays were also utilized to detect specific changes as a result of the induced mutations. Mass spectroscopy was used to analyze the proteins.
Also, specific biotin-based assays (BioID) were used to locate proteins of interest and study the different proteins in the functional parts of the SARS-CoV-2 NSP1 protein.
Importantly this assay helped the study team understand the different parts of the NSP1 protein engaged in different antiviral defense mechanisms. For all these experiments, the researchers used Human HEK293T cells as hosts.
The study team found that stem loop-1 (SL1), which is a hairpin structure in single-stranded RNA that helps the viral mRNA retain its structure and continue specific enzymatic interactions, is both necessary and sufficient to escape NSP1-mediated repression.
Subsequently mutagenesis was used to isolate 3 specific cytosine proteins, which is one of the four nucleic acid bases of RNA, that were crucial to evasion of host defense mechanisms.
Interestingly upon further analysis of the protein by BioID, NSP1 was found to block host translation by inserting its C-terminal domain into the mRNA entry tunnel on the ribosomal 40S subunit. In doing so, NSP1 blocked ribosomal host-protein synthesis and exported the viral mRNAs into the system. An artificially constructed non-functional mutant KH164AANSP1 could not replicate the mechanism of the wild-type NSP1 as effectively.
Typically, when a virus attacks the host cells, viral RNA is sensed by host RNA helicases in infected cells, resulting in the activation of the transcription factors ATF2/c-Jun, IRF3/IRF7, and NF-kB. These factors subsequently induce the production of cytokines, including members of the IFN family, which go on bind to their respective receptors and trigger a second wave of cytokine signaling. These two waves upregulate genes that inhibit viral replication.
Significantly the NSP-1 was ultimately able to evade these pathways to prevent the induction of cytokines, thereby helping the virus to spread in the host system.
The study findings show that the wild-type SARS-CoV NSP1 is responsible for binding to host ribosomes and inducing the production of viral mRNA. NSP1 was also found to evade immune responses and defer the release of inflammatory cytokines.
Importantly the first therapeutic target identified in this study is the binding site for NSP1 to the host ribosome, . A second target would involve inducing mutations in the NSP1 region that interacts with the viral leader to evade host antiviral responses, and that in the SL-1 region, targeting the 3 specific cytosine molecules.
Corresponding author, Dr Marina Chekulaeva from the Berlin Institute for Medical Systems Biology, Max Delbrück Center for Molecular Medicine told Thailand
Medical News, “NSP1's fundamental role in viral infections makes it a highly interesting potential target for drugs. The understandings we have gained of the mechanisms underlying its functions suggest three potential points of attack. The most obvious place to interfere is the site of the protein that interacts with the ribosome and blocks the mRNA entry tunnel. This is the defect observed in the KH164AA NSP1 mutant, which fails to interact with ribosome and is nonfunctional. Targeting K164 and H165 with small molecules therefore appear to be a promising strategy that would disrupt the pathogenicity of SARS-CoV-2. Another weak point that could be exploited is the mechanism which viral molecules use to evade NSP1 silencing: the structure that permits NSP1 to interact with the viral leader. There are two possible targets: the regions in either NSP1 or SL1 that permit and are required for this interaction.”
She further added, “The study finds that the R124A mutant, but not WT NSP1, effectively represses the viral reporter points to a vulnerable spot on NSP1 that could be targeted by therapies. On the SL1 side, finding compounds that target three crucial cytosines (C15, C19 and C20), alone or in combination, might hold a great potential for the development of novel SARSCoV-2 therapies.”
It should further be noted that the suppressing of the human host genes and proteins can ultimately have a bearing on the health condition of the infected individual especially in the long term.
For the latest global
Medical News, keep on logging to Thailand Medical News.

